Clinical Implication of Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease
Article information
Abstract
Tolvaptan, a non-peptide arginine vasopressin V2 receptor antagonist, is a newly developed drug to reduce kidney volume and preserve kidney function in autosomal dominant polycystic kidney disease (ADPKD) patients. We aimed to evaluate the descriptive characteristics of patients according to the use of tolvaptan. Also, we tried to find the efficacy of tolvaptan on kidney volume and kidney function. We included patients with ADPKD who visited a tertiary hospital in South Korea during Sep. 2018 and Apr. 2022. The data was acquired from the Electric Medical Records system. A total of 64 patients were included in the study, and there were 33 (51.6%) patients taking tolvaptan during follow-up periods. During 17.8 ± 13.1 months of follow-up periods, estimated glomerular filtration rate (eGFR) changes were 89.4% compared to the baseline eGFR. Although the latest eGFR was lower in patients with tolvaptan (55.9 ± 24.7 mL/min/1.73 m2) than without tolvaptan (68.4 ± 35.1 mL/min/1.73 m2), there was no statistical significance (p = 0.108). We found that the mean change of height-adjusted total kidney volume (HtTKV) was -2.7% based on the baseline HtTKV in patients taking tolvaptan for more than 1-year. Although there was no statistical significance, the mean change of HtTKV was the highest in patients with 1E of Mayo classification (-4.3%). To anticipate the solid data on the efficacy of tolvaptan in the Asian population, more aggressive efforts are needed to search for suitable patients accompanied by appropriate monitoring over a more extended period.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic kidney disease characterized by the growth of numerous fluid-filled cysts in the kidneys. It leads to progressive kidney enlargement and end-stage kidney disease (ESKD) [1,2]. ADPKD is a ciliopathy that involves abnormal cilia structure and function [3]. There are polycystin-1 and polycystin-2 in the kidney tubular cell protruding primary cilium. It has a role in detecting fluid flow and regulating calcium influx that activates an intracellular calcium signaling pathway [4]. In addition, increased levels of arginine vasopressin levels induced increased intracellular adenosine cyclic monophosphate (cAMP) levels in the distal tubule and collecting duct.
Tolvaptan is a vasopressin receptor antagonist; it selectively blocks the binding of V2 receptors in tubular cells and reduces fluid secretion, cell proliferation, and cyst development by reducing cAMP [5]. Tolvaptan in ADPKD patients was first approved in Japan in 2014 based on a phase 3 clinical trial (TEMPO 3:4) from 2007 to 2012 [6]. Additional trials showed positive results for reducing the rate of increased kidney volume with preserved kidney function; it was finally approved to use in Korea in 2019 [7-9]. However, there were rare data for Asian populations, with only 12.6% in the TEMPO 3:4 trial. Moreover, the used dosage of tolvaptan was lower in Japanese compared to the previously reported one [10]. In this regard, a phase 4 clinical trial has been performed in Korea.
Tolvaptan usually is prescribed in patients defined with the rapid progressor defined by rapidly decreased estimated glomerular filtration rate (eGFR) or Mayo classification 1C-1E. However, there are several hurdles to prescribing the tolvaptan, even in patients showing rapid progression. Aquaretic symptoms such as frequent urination and the requirement of a large amount of water intake were the most common cause of discontinuation of the drug [6]. In addition, the risk of hepatotoxicity required frequent monitoring by blood tests with monthly visits to the hospital. Therefore, before evaluating the hard outcome, we tried to search out the descriptive characteristics between patients with and without tolvaptan treatment in the real clinical field. In addition, this study aimed to evaluate the short-term changes in total kidney volume (TKV) and renal function in patients with tolvaptan.
Materials and Methods
Study populations
A subject who visited Keimyung University Dongsan Hospital during Mar. 2022 and May. 2022 with ADPKD was initially evaluated in this study. We included patients with age ≥ 18 years old with follow-up periods over 3 months. ADPKD was initially screened by the diagnostic code of Q612 based on the International Classification of Diseases 10th version. We confirmed the disease based on the computed tomography (CT) image with a family history. We defined patients with rapid progression as a Mayo Clinic image classification of 1C, 1D, or 1E. Among whole populations, patients with rapid progression, eGFR ≥ 30 mL/min/1.73 m2, with informed consent were candidates for tolvaptan treatment.
Clinical data acquisition
We obtained anthropometric data, laboratory data, and image results using electric medical records. Kidney function was defined by serum creatinine-based eGFR calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation [11]. We measured TKV based on the CT image finding using the ellipsoid equation [12]. TKV was adjusted by height (HtTKV), and it was divided into 5 classifications using Mayo classifications.
Statistical analysis
We compared the baseline characteristics of patients with and without tolvaptan treatment. We used the student t-test and Chi-square test for comparing the two groups with and without using tolvaptan. Also, in patients with tolvaptan treatment, we evaluated the change of TKV with 1-year follow-up CT findings. In comparing the groups according to the change of TKV, we used Mann–Whitney U-test. Continuous variables were represented as the mean and standard deviation in cases that followed a normal distribution and the median with an interquartile range in cases without normal distribution. The percent change of eGFR and TKV was calculated by dividing the latest result by the initial result. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Window, Version 23.0 (IBM Corp., Armonk, NY, USA).
Ethical consideration
The study was approved by the institutional review board of Keimyung University Dongsan Hospital (DSMC 2021-09-018). This study was performed based on the retrospectively reviewed database; thus informed consent for the study was waived.
Results
Study populations
A total of 64 patients were included in the study, and there were 33 (51.6%) patients taking tolvaptan during follow-up periods. The mean age was 44.6 years old, and 62.5% of patients were male. Total kidney volume was 2070.7 mL, and it was significantly higher in patients with tolvaptan (2,280.3 mL) than without tolvaptan (1,847.6 mL). Most patients were included in the rapid progressor with Mayo class 1C-E. Baseline kidney function was similar between the two groups, and the mean eGFR was 68.0 ± 28.8 mL/min/1.73 m2. A comparison of laboratory results between patients with and without tolvaptan was demonstrated in Table 1.
Changes in kidney function according to the use of tolvaptan
During 17.8 ± 13.1 months of follow-up periods, the mean percent changes of eGFR were 89.4% compared to the baseline eGFR. The mean decrease of eGFR was 6.1 mL/min/1.73 m2. According to the use of tolvaptan, mean follow-up periods were 13.6 ± 8.3 months and 22.2 ± 15.7 months in subjects with and without tolvaptan, respectively (p = 0.007). Although the latest eGFR was lower in patients with tolvaptan (55.9 ± 24.7 mL/min/1.73 m2, n = 33) than without tolvaptan (68.4 ± 35.1 mL/min/1.73 m2, n = 31), there was no statistical significance (p = 0.108). The distribution of eGFR was wider in patients without tolvaptan, and each patient showed a different change in eGFR during follow-up periods (Fig. 1). Mean difference of eGFR during follow-up periods were 7.2 ± 6.7 mL/min/1.73 m2 and 4.9 ± 9.9 mL/min/1.73 m2 in patients with and without tolvaptan, respectively.
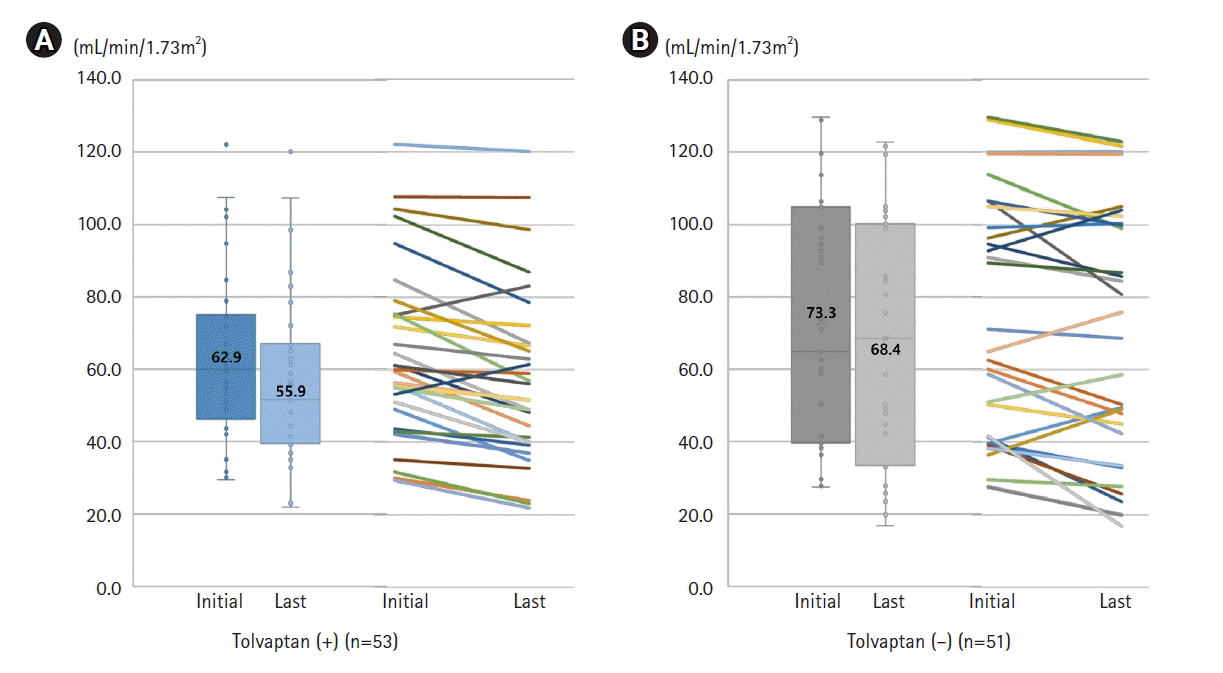
Changing in eGFR between initial and latest visit in patients (A) with and (B) without tolvaptan. Mean follow-up periods were 13.6 ± 8.3 months and 22.2 ± 15.7 months in subjects with and without tolvaptan, respectively. The number in box plot was mean value of eGFR. Each line located in right side of box plot demonstrated eGFR changing in each patient. eGFR, estimated glomerular filtration rate.
Among the subjects who were followed up over 1-year, changes in kidney function were comparable between the patients with tolvaptan (63.9 ± 32.7 mL/min/1.73 m2, n = 15) and without tolvaptan (70.7 ± 38.1 mL/min/1.73 m2, n = 21) (p = 0.580).
Effect of tolvaptan for changing in TKV
Among 33 patients taking tolvaptan, 15 patients were follow-up over 1 year. There were 10 patients with decreased TKV at 1-year follow-up CT compared to the baseline image. The mean percent change of TKV was 97.8%, and the minimum and maximum were 88.9% and 108.5%, respectively. The distribution of changes in TKV and HtTKV was different in each patient (Fig. 2).
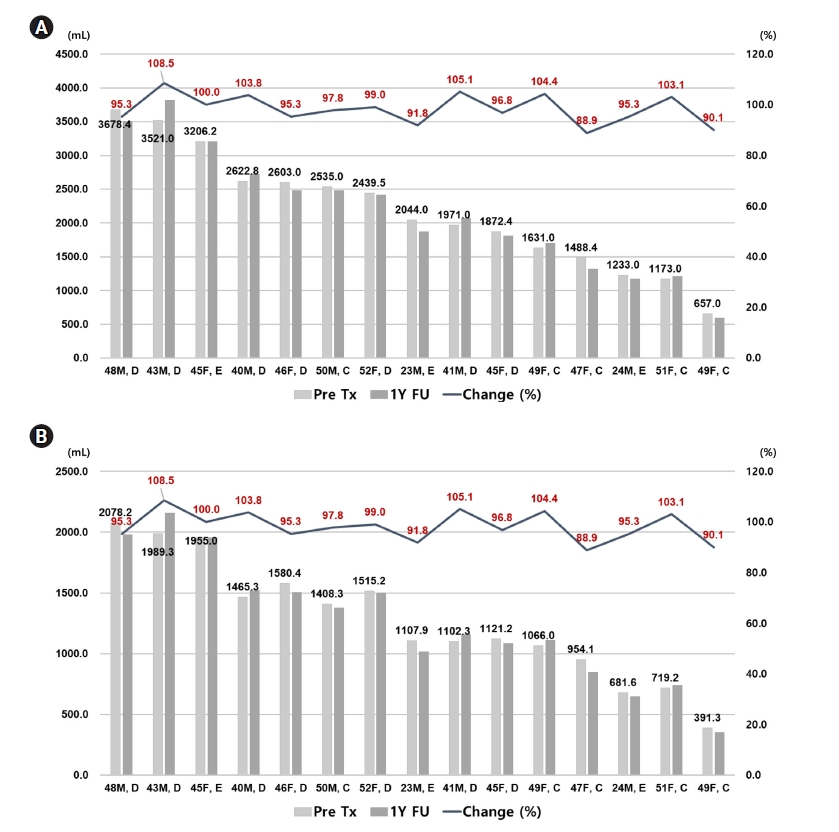
Chainging in (A) total kidney volume (TKV) and (B) height adjusted total kidney volume (HtTKV) in each patient with tolvaptan treatment over 1 year. Left-sided axis and right-sided axis showed kidney volume and percent change of kidney volume, respectively. Light grey and dark grey bar represents initial and 1-year kidney volume in each patient. The number located on the top of light grey bar shows the kidney volume measured at first visit. Line above the bar graph showed percent change of kidney volume. The light grey colored bar represents the TKV (A) and HtTKV (B) at the time before initiation of tolvaptan. The dark grey colored bar represents the TKV (A) and HtTKV (B) at the time of 1-year follow-up state.
In comparing the characteristics between patients with increased TKV and decreased TKV, there were more patients with larger baseline TKV with lower baseline eGFR, but there was no statistical significance (Table 2).
Cases with discontinuation of tolvaptan
A total of 4 patients discontinued tolvaptan in this study. Three of four patients took tolvaptan for 2 years, and they decided to stop to take because of the fatigue and uncomfortable lifestyle. All three patients were female, and they have willing to restart taking medicine within a few months. One of four patients showed severe hepatotoxicity with increasing aspartate aminotransferase (AST) and alanine aminotransferase (ALT) up to 240 mg/dL and 550 mg/dL, respectively. After 2 month-later of discontinuation of tolvaptan, liver function was finally normalized.
Discussion
We found that patients with tolvaptan treatment showed larger TKV than patients without tolvaptan. During 17.8 months, patients experienced 6.1 mL/min/1.73 m2 of decreased eGFR, and there was no statistical difference according to the treatment with tolvaptan. Tolvaptan has the effect of decreasing the TKV in 60% of patients taking tolvaptan over 1 year. Although there was 1 patient suffered from severe hepatotoxicity after taking tolvaptan, it was finally recovered, and he has maintained a stable kidney and liver function. The aquaretic symptom is usually regarded as a critical hurdle for starting tolvaptan, but there was no report of discontinuing the drug in this study.
Kidney volume, especially HtTKV, is regarded as a critical indicator for differentiating the prognosis of ADPKD. The Mayo classification for ADPKD was based on the HtTKV; it was divided into five classes, 1A to 1E. Patients included in classes 1C to 1E are usually expected to progress rapidly to ESKD [12]. The frequency of ESKD at 10-year was significantly increased from class 1A (2.4%) to 1E (66.9%). Based on the result of the renal survival based on the Mayo classification, patients with class 1C-1E were sub-classified as those who have rapidly progressive disease. Moreover, the efficacy of tolvaptan was also different according to the Mayo classification [13]. As a result, tolvaptan was only approved to use in patients categorized into the rapid progression in Korea. In this study, all patients with tolvaptan showed Mayo classification 1C-1E.
The rate of increase of TKV is closely associated with the Mayo classification. Patients with Mayo class 1C, 1D, and 1E were expected to increase in HtTKV by around 3, 5, and > 6% annually [14]. However, tolvaptan significantly influenced the rate of increase in HtTKV; it decreases the size by 0.1 to 0.2% according to Mayo classification 1C to 1E [15]. In this study, we found that the mean change of HtTKV was -2.7% based on the baseline HtTKV. Although there was no statistical significance, the mean change of HtTKV was the highest in patients with class 1E (-4.3%). A more extensive dataset with ADPKD patients is needed to improve the quality of statistical significance.
Baseline kidney function is another critical factor in predicting kidney outcome. The earlier start of tolvaptan in patients with preserved kidney function has been expected to delay the time of renal replacement therapy more. On the contrary, the efficacy of tolvaptan was prominent in patients with chronic kidney disease (CKD) stage 2, 3 [16]. In this regard, we usually prescribe tolvaptan to patients with eGFR ≥ 30 and < 90 mL/min/1.73 m2. In this study, most patients with tolvaptan showed stage 3 CKD. The mean percent change of eGFR incrementally increased from 5.0%, 9.7%, to 13.3% in CKD stages 1, 2, to 3, respectively. Decreases in eGFR was more prominent in subjects with tolvaptan (7.9%, 11.3%, 13.5% in CKD stages 1, 2, and 3) than without tolvaptan (3.9%, 15.2%, 30.2% in CKD stages 1, 2, and 3) irrespective of stage CKD. However, after adjusting the time-interval, annual decreases in eGFR were smaller in subjects with tolvaptan (7.9%, 13.6%, 15.4% in CKD stages 1, 2, and 3) than without tolvaptan (8.2%, 11.6%, 29.2% in CKD stages 1, 2, and 3). Because of the small number of patients included in the study and short-term follow-up duration, it was hard to evaluate the significance of tolvaptan on kidney function. In addition, the absolute latest eGFR was lower in subjects with tolvaptan even though there was no statistical significance. Considering the pharmacological effect of tolvaptan, it could be due to the effect of tolvaptan on suppressing glomerular hyperfiltration. Therefore, to compare the exact effect of tolvaptan on kidney function, it needs to have a wash-out period before the evaluation.
Aquaretic symptom such as polyuria, nocturia, thirst, and polydipsia is a representative side effects of tolvaptan. These symptoms were a significant reason to discontinue the drug in the TEMPO 3:4 clinical study. However, with repetitive education and periods of adjustment, the adherence to the medication was improved with a decreased rate of discontinuation in the TEMPO 4:4 clinical study. Most patients included in this study suffered from aquaretic symptoms, but no one discontinued the drug due to these symptoms. Therefore, we also suggest that assertive education and counseling about these symptoms need to be performed before starting the medication.
Tolvaptan has been associated with idiosyncratic and reversible elevations of blood AST and ALT with infrequent cases of concomitant elevations in total bilirubin [17]. The incidence was low, and most patients completely recovered from hepatotoxicity in previously reported clinical trials [6,8,9]. Nevertheless, one case showed severe injury requiring liver transplantation in Japan [18]. Among 33 patients with tolvaptan, one patient permanently discontinued the medication due to hepatotoxicity in this study. Considering the patient’s clinical characteristics without any risk factors for hepatotoxicity such as hepatitis B, hepatitis C, and fatty liver disease, it was hard to expect this event. Therefore, regular follow-up of liver function test is strongly recommended in patients taking the drug.
In this study, there were 27 patients with ADPKD mayo class 1C-1E among the group without tolvaptan. Following the clinical management flow, for all subjects who are eligible to start tolvaptan, we assertive recommend taking tolvaptan. Nevertheless, there were several concerns not to start tolvaptan. First, the use of tolvaptan has been permitted for only subjects with eGFR ≥ 30 and < 90 mL/min/1.73 m2. Therefore, more than half of the subjects included in the group without tolvaptan could not take tolvaptan irrespective of TKV. Second, aquaretic symptoms make one hesitate to take tolvaptan, especially for subjects who have hurdles to drinking enough water and going to the bathroom frequently. In this regard, the effort to educate with practical application and extend the criteria based on the political policy would be warranted.
Unfortunately, there is still no suitable way to cure ADPKD. The chance to use tolvaptan is a meaningful challenge in patients with ADPKD, even with lots of hurdles to experience during the treatment periods. This study could represent descriptive characteristics of patients with and without tolvaptan. Also, we provided the result of the effect of tolvaptan on change of TKV and kidney function with additive information on side effects. However, there were several limitations to be discussed in this study. It was a retrospective single-center study. The number of included patients was too small to evaluate the statistical significance. In addition, we could not figure out the clinical outcome with a relatively short-term follow-up period. It requires a prospective study with a large number of participants with more extended follow-up periods.
Tolvaptan could be the only option to reduce the rate of increase of TKV and preserve kidney function in ADPKD. However, to expect better outcomes, more attention and effort to excavate suitable patients based on a proper monitoring process.
Notes
Conflict of interest
All authors declare no conflicts-of-interest related to this article.

